Overview
Open-Innovation-Type R&D Consortium to develop innovative medical devices by Accademia-Industry-Government Collaboration
Index of Research Content
-
University collaborating with industries to jointly develop innovative medical devices that meet unmet clinical needs.
-

Sharing data obtained during R&D with industries; University use it for scientific activities and industries for further improvement.
-

Utilizing a complete set of 3D equipment to make our own rapid prototype withstanding bench testing and pre-clinical testing.
-

Inputting customer’s needs that Sales Companies have at early R&D stages to effectively commercialize products appealing to wide range of users.
-
Providing training for Accademia-Industry collaborator by accepting human resource from companies working on R&D on medical devices at clinical training, simulation settings and animal testing facilities.
Content of Research
A research complex that “makes manufacturing science” through unique academic approach.
At the Department of Next Generation Endoscopic Intervention; we are collaborating with many companies through the open innovation community system and using long accumulated know-hows of Academia-Industry Collaboration, we are performing R&D of innovative medical devices needed to achieve next generation minimally invasive diagnosis and treatment. Based on the specific unmet medical needs given by doctors, companies will be organized into subgroups to work from basic research stage through up to pre-clinical / clinical stages for a speedy social implementation.
Throughout our experience in Academia-Industry and Medical & Engineering Collaborations, in order to implement “truly needed medical devices” to the society, we have realized that it is important to learn the needs from the clinical field more than the technology. We also have learnt that relentless modification is necessary after products are placed on the market that mid to long term feedbacks from the users at the clinical field are extremely important for the optimization.
Furthermore, leading many R&D projects, we think the process of development does not just come down to Manufacturing, it is the Academic with latest scientific data and findings that we, doctors as the “end-users” of medical devices should proactively send to the society. We believe that sharing our findings is one of the important missions to offer as an academia of a joint research department.
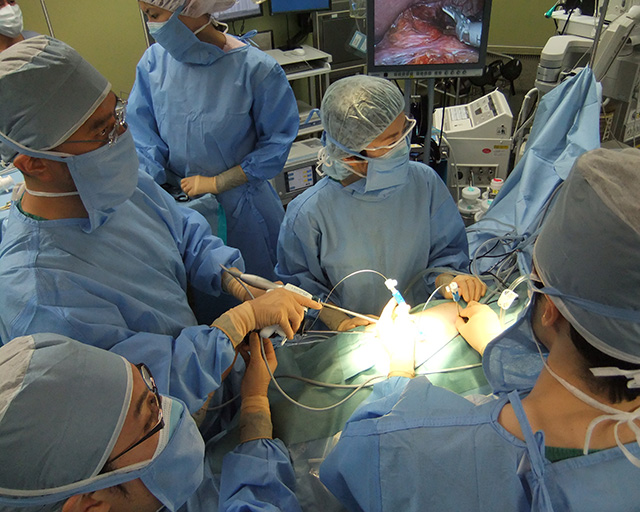
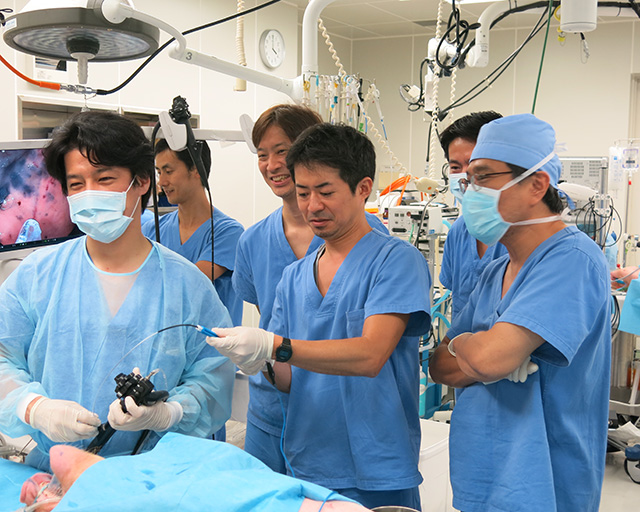
For example, we collaborate with domestic and foreign companies with necessary basic technologies need for R&D of medical devices such as plastic molding, processing technology, metal processing, polymer science, precision chemistry and so on; and the government supports by providing subsidy; we will strive to jointly develop innovative medical devices for the next generation diagnosis and treatment, and by spinning off the innovative medical devices to current diagnosis / treatment, standardize the current technically difficult procedures and make them widely feasible.
These activities have been well regarded not only by participating companies but also by government authorities. The competitive funding (external funding) we have secured so far has come from sources such as the Ministry of Health, Labour and Welfare, the Ministry of Economy, Trade and Industry, and the Ministry of Education, Culture, Sports, Science and Technology. We are currently carrying out projects supported by public funding.
We will continue to collaborate with medical doctors at the clinical field to deliver our ongoing projects, speedy commercialization of our developing products at its prototype stage, security of industrial property rights and to summarize and present scientific data.

